MSG
Manufactured vs Natural Glutamic Acid
Ingredient Names Used to Hide MfG

MSG in Agriculture
MSG can be sprayed on crops without restriction.
In 1998, the U.S. Environmental Protection Agency (EPA) approved virtually unrestricted use of MSG for use on growing produce. The rule, which became effective on February 6, 1998, was summarized as follows in the Federal Register:
ENVIRONMENTAL PROTECTION AGENCYIn other words, MSG can be sprayed on crops without restriction, and with no limit on the amount of MSG that can remain in or on fruits, vegetables, grains, seeds, and nuts when brought to market.
40 CFR Part 180
[OPP-300598; FRL-5764-4]
RIN 2070-AB78
Glutamic Acid; Pesticide Tolerance Exemption
AGENCY: Environmental Protection Agency (EPA).
ACTION: Final rule.
-----------------------------------------------------------------------
SUMMARY: This rule establishes an exemption from the requirement of a tolerance for residues of the biochemical glutamic acid in or on all food commodities, when applied as a plant growth and crop yield enhancer in accordance with good agricultural practices. This exemption was requested by Auxein Corporation.DATES: This regulation becomes effective February 6, 1998. Objections and requests for hearings must be received by EPA on or before March 9, 1998.
The EPA's approvals of use of MSG in agriculture are simple, straightforward, and in violation of the Federal Food, Drug, and Cosmetic Act.
In reviewing the application of Auxein Corporation (now Emerald BioAgriculture) for use of processed free glutamic acid in a spray to be applied to crops as they grow, the EPA failed to conform to the requirements of the Federal Food, Drug and Cosmetic Act, which require, in part, that the EPA review any proposed action for validity, completeness, reliability, and relationship to human risk. The EPA also ignored Executive Order 13045 which requires government agencies to consider available information concerning the variability of the sensitivities of major identifiable subgroups of consumers, including infants and children.For example, Auxein Corporation sent the EPA 14 industry-sponsored toxicological studies from the literature, all done in the 1970's, but failed to mention hundreds of studies in the literature that refuted those 14 studies. Auxein Corporation even failed to send the EPA independent studies that appeared in the same book(s) as the industry-sponsored studies sent to the EPA. For example, although processed free glutamic acid causes brain lesions and neuroendocrine disorders in infant animals, this special hazard faced by infants was ignored by Auxein Corporation. It would appear that Auxein Corporation restricted its consideration of "available information" to information made available by the glutamate industry; and the EPA, even after having been sent abstracts from other "available information," has not challenged the Auxein Corporation applications. A more complete discussion of the shortcomings of the EPA approvals granted to Auxein Corporation were submitted to the EPA by the Truth in Labeling Campaign.
Questions about the safety of spraying processed free glutamic acid on plants and into the environment were raised by the Truth in Labeling Campaign and by individual consumers. The EPA has refused to address those concerns. The EPA, and, in particular, EPA spokesperson Dr. Janet Andersen, has failed to respond to allegations that in approving the spraying of processed free glutamic acid, the EPA failed to consider the reliability, validity, and completeness of the Auxein Corporation application or comply with Executive Order 13045 entitled Protection of Children from Environmental Health Risks and Safety Risks, except to say that the EPA had complied with executive order 13045. Moreover, while responding to letters that asked direct questions of the EPA, Andersen failed to respond to most, if not all, of the direct questions contained in those letters.
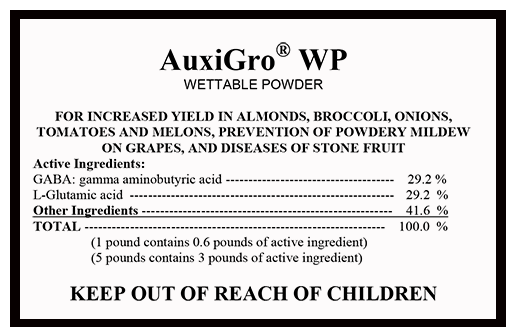 AuxiGro WP Metabolic Primer (AuxiGro), the first MSG-laced plant "growth enhancer" to hit the market, was approved for spraying on every crop we know of, with no restrictions on the amount of processed free glutamic acid (MSG) that may remain in and/or on crops when brought to market. Even before consumers had an inkling that crops were being sprayed, the Truth in Labeling Campaign received reports that MSG-sensitive consumers had gotten sick from head lettuce and potatoes – two crops that the EPA had been approved for experimental (and undisclosed) use of AuxiGro.
AuxiGro WP Metabolic Primer (AuxiGro), the first MSG-laced plant "growth enhancer" to hit the market, was approved for spraying on every crop we know of, with no restrictions on the amount of processed free glutamic acid (MSG) that may remain in and/or on crops when brought to market. Even before consumers had an inkling that crops were being sprayed, the Truth in Labeling Campaign received reports that MSG-sensitive consumers had gotten sick from head lettuce and potatoes – two crops that the EPA had been approved for experimental (and undisclosed) use of AuxiGro.
Federal Register notices chronicling the application and approval of processed free glutamic acid were at one time available on the Web, via GPO Access, the Federal Register. If you have interest, see our report here.
Application for approval of use of AuxiGro was made to the EPA in 1997. Testing of the product was also approved in that year, and many test crops sprayed with AuxiGro were brought to market without notifying consumers. Glutamic acid was granted an exemption from establishment of a tolerance limit in January, 1998. AuxiGro was also approved for use on a number of crops in January, 1998, and approved for use on other crops later. No announcement of these approvals was made in the Federal Register.
Due to a technical glitch in the system, Auxein Corporation came to need one more approval to make their California registrations work. They were asking for AuxiGro to be approved for use as a fungicide in California, but the EPA had only approved AuxiGro for use as a pesticide produce or plant growth enhancer. And when application was made for this addition to their approvals, the application was brought to our attention; and the Truth in Labeling Campaign filed a formal protest to this approval of AuxiGro. The Formal Objection of the Truth in Labeling Campaign was filed on August 16, 2001 with the EPA.
By law, formal objections filed in a timely manner must be responded to within six months. Also, by law (we were told) even though the Final Rule had not been promulgated, this additional use of AuxiGro would be considered approved unless and until the EPA determined that it should be otherwise. In July, 2004, we received a conference call from Dr. Andersen and a number of other EPA players, including an EPA lawyer -- a "courtesy call" telling us that our objections had been discounted and that the Final Rule allowing use of AuxiGro as a fungicide would be published shortly in the Federal Register.
What's wrong at the EPA?
Neither the EPA nor Janet Andersen, Ph.D., director of the Biopesticides and Pollution Prevention Division (BPPD), are stupid. Rather, all evidence would appear to suggest that the EPA, which is charged with protecting the health of Americans, says it is protecting the health of Americans, when in fact the EPA acts to protect the bottom line of big business. Don't think for a moment that MSG is the only toxin unleashed on the American public by the EPA. Let the words "methyl parathion," "DDT," GMOs and glyphosate, jog your memory.The EPA, in granting the chemical referred to as "L-glutamic acid" an exemption from the requirement of a tolerance for residues of "L-glutamic acid" on all food commodities when applied/used in accordance with good agricultural practices (thereby allowing unrestricted amounts of processed free glutamic acid (MSG) residue to remain in and on any and all food crops that come under the EPA's jurisdiction) violated Section 408(c)(2)(A)(i), Section 408(c)(2)(ii), Section 408(c)(2)(B), and Section 408(b)(2)(D) of the Federal Food, Drug, and Cosmetic Act.
Neither "L-Glutamic Acid and Gamma Aminobutyric Acid; Exemptions from the Requirement of a Tolerance; Final Rule" (Federal Register June 21, 2001) nor "Glutamic Acid; Pesticide Tolerance Exemption; Final Rule" (Federal Register January 7, 1998), which preceded it, met the criteria established by law for granting exemptions from the restriction of a tolerance.
How did spokesperson Andersen excuse the fact that the EPA approved processed free glutamic acid for use in an EPA approved spray? First, said Andersen, the free glutamic acid used in the spray is naturally occurring, and it's 99.3 per cent pure pharmaceutical grade L-glutamic acid. Yet, in admitting that the free glutamic acid in AuxiGro is not 100 per cent pure L-glutamic acid, and that it is pharmaceutical grade, Andersen contradicted herself, and actually made the point that 1) if the free glutamic acid used in AuxiGro were truly natural, it wouldn't be "pharmaceutical grade;" and 2) if the free glutamic acid used in AuxiGro were truly natural it would be 100 per cent, not 99.3 per cent pure L-glutamic acid.
Andersen said something else very interesting. She said that the EPA is well aware of the fact that MSG causes adverse reactions. However, when Andersen used the term "MSG" she was referring to the one food ingredient called "monosodium glutamate," and not to the free glutamic acid in "monosodium glutamate" that causes adverse reactions. Failure to define terms, as Anderson did (and does) so handily, is both deceptive and misleading.
What Andersen did is very clever. What she said makes no sense at all. No one has ever claimed that the processed free glutamic acid in AuxiGro comes out of a box labeled "monosodium glutamate." So for her to say it doesn't, is meaningless. On the other hand, the claim has been made that the free glutamic acid in AuxiGro will cause the same brain lesions, neuroendocrine disorders, adverse reactions and other diverse disease conditions that are caused by the free glutamic acid in "monosodium glutamate" and the other food additives that contain processed free glutamic acid. That claim is true, but Andersen does not address it. How do you refute someone who ignores legitimate questions and spews out irrelevant statements as though they pertained to your legitimate questions? You don't. The EPA defense of its approval of use of processed free glutamic acid in plant "growth enhancers" and its registration of AuxiGro has two parts to it: 1) ignoring those who question EPA actions, and 2) making the irrelevant statement that AuxiGro does not contain MSG (monosodium glutamate).
Neither Andersen nor anyone else at the EPA ever addressed the criticism that approvals given by the EPA to allow the use of free glutamic acid and the product AuxiGro were inappropriate.
The EPA, which approved the use of processed free glutamic acid in plant "growth enhancers," made a grievous error. But instead of recognizing and remedying that error once it was pointed out to them, the EPA began a cover-up. That cover-up included use of ambiguous words and phrases, half-truths, and downright lies told to consumers. The cover-up continued (and continues still) with a variation of those ambiguous words and phrases, half-truths, and downright lies told to legislators who inquire about spraying MSG into the environment.
Objections to the use of MSG in sprays to be used on growing crops were filed with both the EPA and the State of California by the Truth in Labeling Campaign.
The state of California, which often has more stringent requirements than the EPA, also approved MSG for use in agricultural products.
In May, 1999, spraying MSG on wine grapes (calling the spray a fertilizer) was approved by the California Department of Food and Agriculture (CDFA). Steven D. Wong, Branch Chief, Agricultural Commodities and Regulatory Services (916/654-0574) told us that there was no demonstration that use according to label directions would present a significant health hazard to workers, consumers of products grown with the aid of the MSG-containing product, or to the general public. To have a product approved for use as a fertilizer in California, a company need do little more than make application.
In April, 2000, and again in July, 2001, spraying MSG on wine grapes (calling it a fungicide) was approved by the California Department of Pesticide Regulation (CDPR). Barry Cortez, Branch Chief, CDPR, first told us that the CDPR would only turn down a product if it appeared to be ineffective, and AuxiGro didn't appear to be ineffective. After reading the law, however, we found that according to Section 12825 of the Food and Agricultural Code:
"Pursuant to Section 12824, the director,...may cancel the registration of, or refuse to register, any pesticide:And AuxiGro meets each of those three criteria.
(a) That has demonstrated serious uncontrollable adverse effects either within or outside the agricultural environment.
(b) The use of which is of less public value or greater detriment to the environment than the benefit received by its use.
(f) Concerning which any false or misleading statement is made or implied by the registrant or his or her agent, either verbally or in writing, or in the form of any advertising literature."
Does spraying processed free glutamic acid onto crops and into the environment pose a problem? We think it does. Does applying processed free glutamic acid to the soil pose a problem? Yes, we think it does; and we have made our thoughts known. On June 8, 1999, even though we knew full well that the glutamate industry had been generous in funding chemists and food scientists at UC Davis, and that those chemists and food scientists and/or their friends would probably be asked to evaluate our comments, we first formally presented our concerns to the CDPR.
It was not until the spring of 2001, however, that we found that AuxiGro contains more awful ingredients than the so called "L-glutamic acid” – which is actually processed free glutamic acid made up of L-glutamic acid, D-glutamic acid, pyroglutamic acid and assorted other unwanted by-products of production. AuxiGro, we learned from government documents, contains hydrolyzed casein (milk) protein, a substance known to have caused the death of milk-sensitive children who consumed minute quantities of milk protein hidden in processed food. AuxiGro, we learned from other government documents, also contains carcinogens.
As of August 20, 2004, California had already approved the following crops for spraying with MSG: ALMOND, APRICOT, CANTALOUPE, CHERRY, GRAPES MELONS, NECTARINE, ONION (DRY, SPANISH), WHITE PEACH, PLUM (INCLUDES WILD PLUMS), PRUNE, TOMATO, TOMATOES FOR PROCESSING, WATERMELONS
On July 9, 2004, California proposed to also allow cole crops to be sprayed with MSG. Cole crops include BRUSSELS SPROUTS, CABBAGE, CAULIFLOWER, KALE, COLLIARDS, TURNIPS, RUTABAGA, BROCCOLI, MUSTARD, WATER CRESS, KOHLRABI.
Objections were filed with both the EPA and the State of California by the Truth in Labeling Campaign. Both agencies responded with words we have come to expect from the glutamate industry.
What's wrong with using glutamic acid, an amino acid found in protein, as a spray on crops?
-
In protein, amino acids are found in balanced combinations. Use of free glutamic acid as a spray on crops throws the amino acid balance out of kilter.
- It's not the glutamic acid found in protein that is being sprayed on crops, it's a synthetic product. The spray being used most widely is called AuxiGro. The "free glutamic acid" or so called "L-glutamic acid" component being used by its manufacturer, Emerald BioAgriculture, contains L-glutamic acid, an amino acid found in protein; but it also contains D-glutamic acid, pyroglutamic acid, and other chemicals referred to in the industry as "impurities." The free glutamic acid used in AuxiGro is processed free glutamic acid. It is manufactured -- in chemical plants -- where certain selected genetically engineered bacteria -- feeding on a liquid nutrient medium -- excrete the free glutamic acid they synthesize outside of their cell membrane into the liquid medium in which they are grown. In contrast, the free glutamic acid found in protein, and the free glutamic acid involved in normal human body function, are unprocessed and contain no impurities.
- No one knows what the long-term effects of spraying processed free glutamic acid on crops will be.
- It cannot be denied that the processed free glutamic acid (MSG) will be absorbed into the body of the plant and into the fruit, nuts, seeds, or vegetable it produces. If it were not, the plant would not be stimulated to grow. Emerald BioAgriculture, the EPA, and the CDPR have all refused to address this issue.
- The fact that there will be residue left on crops has not been disputed by Emerald BioAgriculture. But no study of either the amount of that residue, or the least amount of processed free glutamic acid needed to cause a reaction in an MSG-sensitive person, has ever been done. "It should wash off" doesn't mean it will wash off. "It seems unlikely that such a small amount would cause a reactions" doesn't mean that a small amount will not cause a reaction or have long term health effects.
- Free glutamic acid is known to be toxic to the nervous system. But the neurotoxic effects that processed free glutamic acid will have on animals that consume the plants on which it is sprayed - effects over and above any effects caused by external glutamic acid residue - have never been evaluated. Neither are there data on the effects that spraying processed free glutamic acid will have on drinking water.
- Consider, also, that children are most at risk from the effects of processed free glutamic acid. Their undeveloped blood-brain barriers leave them most at risk from exposure to processed free glutamic acid. It has been repeatedly demonstrated that infant animals fed processed free glutamic acid when young develop neuroendocrine problems such as gross obesity, stunted growth, and reproductive disorders later in life, and that they also develop learning disabilities. Emerald BioAgriculture did not address that particular safety issue in its application to the EPA.
- No one knows how little glutamic acid is needed to kill a single brain cell or to trigger an adverse reaction.
- Free glutamic acid is a neurotransmitter. It causes nerves to fire, carrying nerve impulses throughout the nervous system.
- Free glutamic acid is a neurotoxin. Under certain circumstances, free glutamic acid will cause nerves to fire repeatedly, until they die.
- Processed free glutamic acid kills brain cells. The free glutamic acid ingested by laboratory animals that caused brain lesions and neuroendocrine disorders was very often given in the form of the food ingredient "monosodium glutamate." "Monosodium glutamate" is the name of a particular food additive. Processed free glutamic acid is the toxic component in "monosodium glutamate," just as processed free glutamic acid is a toxic component in AuxiGro.
- The glutamate industry research done in the 1970s that was submitted to the EPA by the Auxein Corporation, that pretended to find that processed free glutamic acid is "safe," has been long refuted by independent scientists. Indeed, at the present time, neuroscientists attempting to develop pharmaceuticals to block the toxic effects of free glutamic acid are using processed free glutamic acid to selectively kill brain cells.
- Processed free glutamic acid causes neuroendocrine disorders in maturing animals that ingest processed free glutamic acid early in life.
- Processed free glutamic acid causes learning disorders in maturing animals that ingest processed free glutamic acid early in life.
- Processed free glutamic acid crosses the placental barrier and causes learning disabilities in animal offspring of dams that ingest it.
- Processed free glutamic acid has access to the brain through the blood-brain barrier, which is not impervious to the unregulated flow of processed free glutamic acid. The blood-brain barrier is immature at birth and may continue to develop up to puberty. In certain areas called the circumventricular organs, the blood barrier is never impervious to the unregulated flow of free glutamic acid. In addition, the blood-brain barrier is easily damaged by such events as high fever, a blow to the head, drug use, stroke, ingestion of processed free glutamic acid, and the normal process of aging.
- The National Institutes of Health recognize glutamic acid as being associated with addiction, stroke, epilepsy, degenerative disorders such as Alzheimer's disease, Parkinson's disease, and ALS, brain trauma, neuropathic pain, schizophrenia, anxiety, and depression.
- For years, free glutamic acid has been produced and used in food additives with names such as monosodium glutamate, sodium caseinate, and hydrolyzed soy protein. In some people, the processed free glutamic acid in food additives causes adverse reactions that include migraine headache, asthma, arrhythmia, tachycardia, nausea and vomiting, depression, and disorientation. The processed free glutamic acid in prescription and non-prescription pharmaceuticals, food supplements, and cosmetics can also cause adverse reactions.
- There are badly flawed industry-sponsored studies that have pretended to find that processed free glutamic acid does not cause adverse reactions. Inappropriate procedures used by the glutamate industry have included limiting subjects to people virtually guaranteed not to be sensitive to processed free glutamic acid, and/or using processed free glutamic acid or other similarly reactive substances in placebos as well as in test material. The Food and Drug Administration (FDA) has based its claim that processed free glutamic acid causes only mild and transitory reactions on those badly flawed industry-sponsored studies.
- Even the EPA admits that the food additive called "monosodium glutamate" causes adverse reactions.
- Even the FDA admits that the food additive "monosodium glutamate" contains processed free glutamic acid.
- Even the FDA admits that many consumers refer to all free glutamic acid as "MSG." Sales literature promoting AuxiGro was once found on the Web sites of Auxein Corporation and Emerald BioAgriculture, but is now long gone. While Federal Register notices included the fact that there is processed free glutamic acid (MSG) in AuxiGro, the sales literature from Auxein Corporation did not mention the fact that their product contains free glutamic acid until the Truth in Labeling Campaign began to broadcast that information. In November, 1999, Auxein added deceptive, misleading, and untrue statements in an elaboration of its Product Page, wherein they essentially make the untrue assertion that the glutamic acid used in AuxiGro is chemically and biologically identical to that found in plants and animals.
Sales literature did (on September 12, 2000), however, contain the following: "PRECAUTIONARY STATEMENTS HAZARDS TO HUMAN AND DOMESTIC ANIMALS – CAUTION"
However, it appears that one MSG-containing product (AuxiGro) is no longer for sale in the American market.
The producer, Emerald BioAgriculture, evidently did not renew its registration with either the EPA or the CDPR. However, in the United States, there are other MSG-containing fertilizer or pesticide products now on the market (hydrolyzed fish protein and hydrolyzed chicken feathers for example), and AuxiGro is being sold in much of the rest of the world. Last time we looked, for example, you could still get your MSG-fix from wine produced in France (yes, France) and Spain – as well as lots of other countries.

